Some substances exist as gases at room temperature oxygen and carbon dioxide while others like water and mercury metal exist as liquids.
Carbon state of matter at room temperature.
Solid liquid or gas the state a given substance exhibits is also a physical property.
Carbon is a solid at room temperature.
Depending on its form carbon has different.
However it can exist in numerous allotropes different structural forms such as graphite diamond or fullerenes e g.
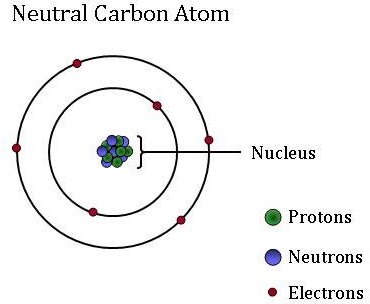
Carbon is a non metal element.
Gases oxygen hydrogen nitrogen and solids carbon phosphorus sulfur and selenium.
Relative atomic mass the mass of an atom relative to that of.
Matter typically exists in one of three states.
Carbon is solid at room temperature.
Although thermodynamically prone to oxidation carbon resists oxidation more effectively than elements such as iron and copper which are weaker reducing agents at room temperature.
Matter and its states.
Although carbon 14 decays into nitrogen 14 through beta decay the amount of carbon 14 in the environment remains constant because new carbon 14 is always being created in the upper atmosphere by cosmic rays.
Density g cm 3 density is the mass of a substance that would fill 1 cm 3 at room temperature.
Carbon is classified as an element in the non metals section which can be located in groups 14 15 and 16 of the periodic table.
The most famous of all fullerenes the.
Carbon exists in different forms including graphite diamond and graphene.
Living things tend to ingest materials that contain carbon so the percentage of carbon 14 within living things is the same as the.
Most of the metals are solids under ordinary conditions i e 25ºc 1 atmosphere of pressure etc with the exception of mercury hg element 80 which solidifies.
Its first four.
In the periodic table above black squares indicate elements which are solids at room temperature about 22ºc those in blue squares are liquids at room temperature and those in red squares are gases at room temperature.
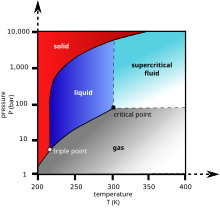
Sublimation the transition of a substance directly from the solid to the gas phase without passing through a liquid phase.
The temperature at which the liquid gas phase change occurs.
At room temperature it is in a solid state.